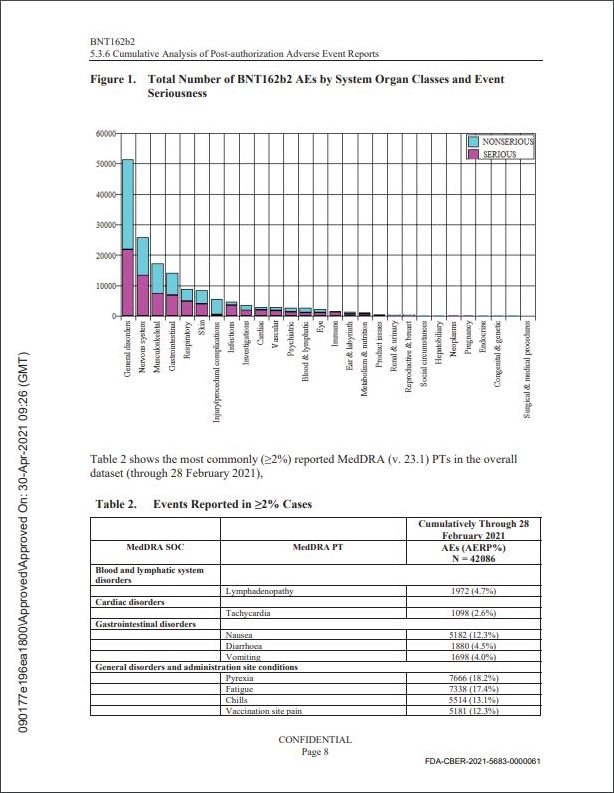
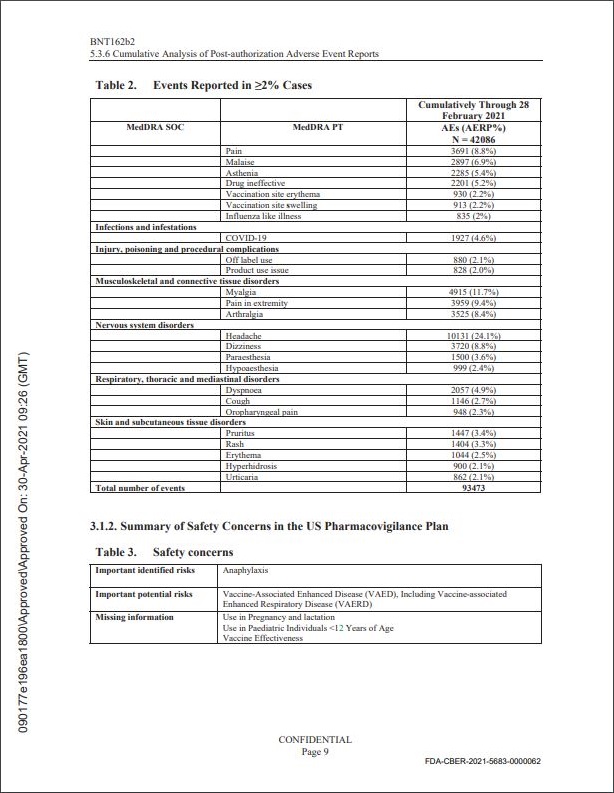
Pfizer vidste allerede 28. februar 2021,
at vi ville se et massivt antal bivirkninger og dødsfald,
helt specifikt 42.086 rapporter indeholdende
158.893 hændelser(herunder 1223 dødsfald).
FDA har frigivet den første omgang af dokumenter, som de har gennemgået, inden de licenserede Pfizers "vaccine". Efter at en gruppe på mere end 30 professorer og videnskabsmænd fra universiteter, herunder Yale, Harvard, UCLA og Brown, anlagde sag i septemberFDA har produceret de første 91+ sider med dokumenter fra Pfizers COVID-19-vaccinefil, og foreslår en gradvis udgivelse på 500 sider om måneden indtil færdiggørelsen af udgivelsen i år 2076.
Et af de fremlagte dokumenter er en kumulativ analyse af rapporter om uønskede hændelser efter autorisation modtaget til og med 28. februar 2021, to en halv måned efter, at vaccinen modtog nødgodkendelse. Dette dokument afspejler uønskede bivirkninger efter "vaccination", både i og uden for USA, frem til den 28. februar 2021.
Pfizer forklarer på side 6, at "På grund af det store antal spontane bivirkninger, der er modtaget for produktet, har Pfizer prioriteret behandlingen af alvorlige sager...", og at Pfizer "også har truffet flere handlinger for at hjælpe med at afhjælpe den store stigning i rapporter om uønskede bivirkninger", herunder "øgning af antallet af dataindtastning og sagsbehandlingskolleger" og "har indsat ca. [antal fjernet fra dokumenet] yderligere fuldtidsansatte."
Hvad angår mængden af indrapporterede bivirkningr, modtog Pfizer i de 2½ måned efter deres nødgodkendelse i alt 42.086 rapporter indeholdende 158.893 hændelser (herunder . De fleste af disse rapporter var fra USA og uforholdsmæssigt involverede kvinder (29.914 mod 9.182 leveret af mænd) og dem mellem 31 og 50 år (13.886 mod 21.325 for alle andre aldersgrupper tilsammen, med yderligere 6.876, hvis alder var ukendt). 25.957 af hændelserne blev klassificeret som "nervesystemlidelser"
Fuld download af dokumenterne
pd-production-111721
5.2-listing-of-clinical-sites-and-cvs-pages-1-41.pdf
5.2-tabular-listing.pdf
5.3.6-postmarketing-experience.pdf
BATES-92_adc19ef-ve-cov-7pd2-wo-eval-sas.txt
BATES-93_tv.xpt
pd-production-120121
Pages-42-289-Section-5.2-listing-clinical-sites-cvs_Part-A.pdf
Pages-42-289-Section-5.2-listing-clinical-sites-cvs_Part-B.pdf
pd-production-121321
BATES-FDA-CBER-2021-5683-0002372_adc19ef-ve-cov-7pd2-eval-sas.txt
BATES-FDA-CBER-2021-5683-0002373_adc19ef-ve-sev-cov-7pd2-eval-sas.txt
BATES-FDA-CBER-2021-5683-0002374_adc19ef-ve-sev-cov-7pd2-wo-eval-sas.txt
BATES-FDA-CBER-2021-5683-0002375_addv-sas.txt
BATES-FDA-CBER-2021-5683-0002376_relrec.xpt
BATES-FDA-CBER-2021-5683-0002377_supppr.xpt
BATES-FDA-CBER-2021-5683-0002378_ta.xpt
BATES-FDA-CBER-2021-5683-0002379_te.xpt
BATES-FDA-CBER-2021-5683-0002380_ti.xpt
CRFs-for-site-1055.pdf
signed-F21-5683-CBER-Dec-13-2021-Response-Letter.pdf
STN-125742_0_0-Section-2.5-Clinical-Overview.pdf
STN-125742_0_0-Section-2.7.3-Summary-of-Clinical-Efficacy.pdf
STN-125742_0_0-Section-2.7.4-summary-clin-safety.pdf
pd-production-122221
Supplemental-Index-12-22-21.pdf
pd-production-123021
pd-production-123021/CRFs-for-site-1081.pdf
pd-production-011822
CRFs-for-site-1096.pdf
pd-production-013122
CRFs-for-site-1128.pdf
pd-production-030122
125742_S1_M1_356h.pdf
125742_S1_M1_3674.pdf
125742_S1_M1_cover.pdf
125742_S1_M1_debarment.pdf
125742_S1_M1_exclusivity-claim.pdf
125742_S1_M1_fast-track-designation.pdf
125742_S1_M1_financial-cert-3454.pdf
125742_S1_M1_financial-cert-bias.pdf
125742_S1_M1_ipsp-agreed-letter.pdf
125742_S1_M1_priority-review-request.pdf
125742_S1_M1_trans-of-oblig.pdf
125742_S1_M1_us-agent-authorization.pdf
125742_S1_M1_userfee.pdf
125742_S1_M1_waiver-req-designated-suffix.pdf
125742_S1_M2_22_introduction.pdf
125742_S1_M2_24_nonclinical-overview.pdf
125742_S1_M2_26_pharmkin-tabulated-summary.pdf
125742_S1_M2_26_pharmkin-written-summary.pdf
125742_S1_M2_27_literature-references.pdf
125742_S1_M2_27_synopses-indiv-studies.pdf
125742_S1_M4_4223_185350.pdf
125742_S1_M4_4223_R-20-0072.pdf
125742_S1_M5_5351_c4591001-ad-hoc-label-tables.pdf
125742_S1_M5_5351_c4591001-fa-interim-compliance-sensitive.pdf
125742_S1_M5_5351_c4591001-fa-interim-compliance.pdf
125742_S1_M5_5351_c4591001-fa-interim-efficacy-response.pdf
125742_S1_M5_5351_c4591001-fa-interim-errata.pdf
125742_S1_M5_5351_c4591001-fa-interim-excluded-patients-sensitive.pdf
125742_S1_M5_5351_c4591001-fa-interim-excluded-patients.pdf
125742_S1_M5_5351_c4591001-fa-interim-lab-measurements-sensitive.pdf
125742_S1_M5_5351_c4591001-fa-interim-lab-measurements.pdf
125742_S1_M5_5351_c4591001-fa-interim-oversight-committees.pdf
125742_S1_M5_5351_c4591001-fa-interim-protocol.pdf
125742_S1_M5_5351_c4591001-interim-mth6-oversight-committees.pdf
125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf
125742_S1_M5_c4591001-A-201114-hiv-preferred-terms.pdf
125742_S1_M5_c4591001-A-adrg.pdf
125742_S1_M5_c4591001-A-bmi-12-15-scale.pdf
125742_S1_M5_c4591001-A-c4591001-phase-1-subjects-from-dmw.pdf
125742_S1_M5_c4591001-A-c4591001-subject-list-for-12-25-immuno-analysis-27jan2021.pdf
125742_S1_M5_c4591001-A-comorbidity-categories.pdf
125742_S1_M5_c4591001-A-first-c4591001-360-participants-enrolled-v1-13aug20-update.pdf
125742_S1_M5_c4591001-A-newlist-c4591001-6k-participants-enrolled-v3-17sep2020.pdf
125742_S1_M5_c4591001-A-report-cci-aids-hiv.pdf
125742_S1_M5_c4591001-A-report-cci-any-malignancy.pdf
125742_S1_M5_c4591001-A-report-cci-cerebrovascular.pdf
125742_S1_M5_c4591001-A-report-cci-chf.pdf
125742_S1_M5_c4591001-A-report-cci-dementia.pdf
125742_S1_M5_c4591001-A-report-cci-diabetes-with-comp.pdf
125742_S1_M5_c4591001-A-report-cci-diabetes-without-comp.pdf
125742_S1_M5_c4591001-A-report-cci-hemiplegia.pdf
125742_S1_M5_c4591001-A-report-cci-leukemia.pdf
125742_S1_M5_c4591001-A-report-cci-lymphoma.pdf
125742_S1_M5_c4591001-A-report-cci-metastatic-tumour.pdf
125742_S1_M5_c4591001-A-report-cci-mi.pdf
125742_S1_M5_c4591001-A-report-cci-mild-liver.pdf
125742_S1_M5_c4591001-A-report-cci-mod-sev-liver.pdf
125742_S1_M5_c4591001-A-report-cci-peptic-ulcer.pdf
125742_S1_M5_c4591001-A-report-cci-periph-vasc.pdf
125742_S1_M5_c4591001-A-report-cci-pulmonary.pdf
125742_S1_M5_c4591001-A-report-cci-renal.pdf
125742_S1_M5_c4591001-A-report-cci-rheumatic.pdf
125742_S1_M5_c4591001-T-S-final-reacto-tables-track.pdf
125742_S1_M5_c4591001-T-S-roadmap.pdf
125742_S1_M5_c4591001-T-S-summary-differences-csr-vs-update.pdf
125742_S1_M5_c4591001-T-S-suppl-arg.pdf
125742_S1_M5_c4591001-T-S-updated-reacto-tlf.pdf
125742_S1_M5_CRF_c4591001-1085-10851018.pdf
125742_S1_M5_CRF_c4591001-1085-10851116.pdf
125742_S1_M5_CRF_c4591001-1085-10851129.pdf
125742_S1_M5_CRF_c4591001-1085-10851216.pdf
125742_S1_M5_CRF_c4591001-1085-10851246.pdf
FDA-CBER-2021-5683-0021888 to -0021901_125742_S1_M5_c4591001-A-P-adae-s091-all-pd2-p3-saf1-sas.txt
/FDA-CBER-2021-5683-0021902 to -0021915_125742_S1_M5_c4591001-A-P-adae-s091-all-pd2-p3-saf2-sas.txt
FDA-CBER-2021-5683-0021916 to -0021929_125742_S1_M5_c4591001-A-P-adae-s092-all-unb-p3-saf-sas.txt
FDA-CBER-2021-5683-0021930 to -0021943_125742_S1_M5_c4591001-A-P-adae-s092-cr-cut-p3x-saf-sas.txt
FDA-CBER-2021-5683-0021944 to -0021963_125742_S1_M5_c4591001-A-P-adae-sas.txt
FDA-CBER-2021-5683-0021964 to -0021980_125742_S1_M5_c4591001-A-P-adae-vax-tier2-p3-saf-sas.txt
FDA-CBER-2021-5683-0021981 to -0022027_125742_S1_M5_c4591001-A-P-adc19ef-sas.txt
FDA-CBER-2021-5683-0022028 to -0022040_125742_S1_M5_c4591001-A-P-adc19ef-ve-cov-7pd2-sg-eval-sas.txt
FDA-CBER-2021-5683-0022041 to -0022053_125742_S1_M5_c4591001-A-P-adc19ef-ve-cov-7pd2-wo-sg-eval-sas.txt
FDA-CBER-2021-5683-0022054 to -0022062_125742_S1_M5_c4591001-A-P-adc19ef-ve-sev-cov-pd1-aai-sas.txt
FDA-CBER-2021-5683-0022063 to -0022135_125742_S1_M5_c4591001-A-P-adce-s010-lr-p3-saf-sas.txt
FDA-CBER-2021-5683-0022136 to -0022325_125742_S1_M5_c4591001-A-P-adce-s020-se-p3-saf-sas.txt
FDA-CBER-2021-5683-0022326 to -0022345_125742_S1_M5_c4591001-A-P-adcevd-sas.txt
FDA-CBER-2021-5683-0022346 to -0022364_125742_S1_M5_c4591001-A-P-adcm-sas.txt
FDA-CBER-2021-5683-0022365 to -0022560_125742_S1_M5_c4591001-A-P-adds-s002-all-p3-rand-sas.txt
FDA-CBER-2021-5683-0022561 to -0022567_125742_S1_M5_c4591001-A-P-adds-sas.txt
FDA-CBER-2021-5683-0022568 to -0022600_125742_S1_M5_c4591001-A-P-adfacevd-sas.txt
FDA-CBER-2021-5683-0022601 to -0022617_125742_S1_M5_c4591001-A-P-admh-sas.txt
FDA-CBER-2021-5683-0022618 to -0022691_125742_S1_M5_c4591001-A-P-adsl-demo-7d-eval-eff-sas.txt
FDA-CBER-2021-5683-0022692 to -0022765_125742_S1_M5_c4591001-A-P-adsl-demo-7d-wwo-eval-eff-sas.txt
FDA-CBER-2021-5683-0022766 to -0022792_125742_S1_M5_c4591001-A-P-adsl-fu-d2-p3-saf-sas.txt
FDA-CBER-2021-5683-0022793 to -0022866_125742_S1_M5_c4591001-A-P-adsl-s005-demo-all-p3-saf-sas.txt
FDA-CBER-2021-5683-0022867 to -0023006_125742_S1_M5_c4591001-A-P-adsl-sas.txt
FDA-CBER-2021-5683-0023007 to -0023031_125742_S1_M5_c4591001-A-P-adsympt-sas.txt
FDA-CBER-2021-5683-0023032 to -0023065_125742_S1_M5_c4591001-A-P-adva-sas.txt
FDA-CBER-2021-5683-0023150 to -0023454_125742_S1_M5_c4591001-A-define.xml
FDA-CBER-2021-5683-0023455 to -0023486_125742_S1_M5_c4591001-A-define-2-0-0.xsl
FDA-CBER-2021-5683-0023490 to -0023491_125742_S1_M5_c4591001-A-bmi-12-15-scale.xlsx
FDA-CBER-2021-5683-0023500 to -0023507_125742_S1_M5_c4591001-A-c4591001-phase-1-subjects-from-dmw.xlsx
FDA-CBER-2021-5683-0023523 to -0023538_125742_S1_M5_c4591001-A-c4591001-subject-list-for-12-25-immuno-analysis-27jan2021.xlsx
FDA-CBER-2021-5683-0023540_125742_S1_M5_c4591001-A-comorbidity-categories.xlsx
FDA-CBER-2021-5683-0023550 to -0023569_125742_S1_M5_c4591001-A-first-c4591001-360-participants-enrolled-v1-13aug20-update.xlsx
FDA-CBER-2021-5683-0023711 to -0023875_125742_S1_M5_c4591001-A-newlist-c4591001-6k-participants-enrolled-v3-17sep2020.xlsx
FDA-CBER-2021-5683-0023878_125742_S1_M5_c4591001-A-report-cci-aids-hiv.xlsx
FDA-CBER-2021-5683-0023895 to -0023915_125742_S1_M5_c4591001-A-report-cci-any-malignancy.xlsx
FDA-CBER-2021-5683-0023919 to -0023921_125742_S1_M5_c4591001-A-report-cci-cerebrovascular.xlsx
FDA-CBER-2021-5683-0023924_125742_S1_M5_c4591001-A-report-cci-chf.xlsx
FDA-CBER-2021-5683-0023926_125742_S1_M5_c4591001-A-report-cci-dementia.xlsx
FDA-CBER-2021-5683-0023928_125742_S1_M5_c4591001-A-report-cci-diabetes-with-comp.xlsx
FDA-CBER-2021-5683-0023930_125742_S1_M5_c4591001-A-report-cci-diabetes-without-comp.xlsx
FDA-CBER-2021-5683-0023932_125742_S1_M5_c4591001-A-report-cci-hemiplegia.xlsx
FDA-CBER-2021-5683-0023936 to -0023937_125742_S1_M5_c4591001-A-report-cci-leukemia.xlsx
FDA-CBER-2021-5683-0023944 to -0023950_125742_S1_M5_c4591001-A-report-cci-lymphoma.xlsx
FDA-CBER-2021-5683-0023955 to -0023958_125742_S1_M5_c4591001-A-report-cci-metastatic-tumour.xlsx
FDA-CBER-2021-5683-0023960_125742_S1_M5_c4591001-A-report-cci-mi.xlsx
FDA-CBER-2021-5683-0023963_125742_S1_M5_c4591001-A-report-cci-mild-liver.xlsx
FDA-CBER-2021-5683-0023965_125742_S1_M5_c4591001-A-report-cci-mod-sev-liver.xlsx
FDA-CBER-2021-5683-0023967_125742_S1_M5_c4591001-A-report-cci-peptic-ulcer.xlsx
FDA-CBER-2021-5683-0023971 to -0023972_125742_S1_M5_c4591001-A-report-cci-periph-vasc.xlsx
FDA-CBER-2021-5683-0023975 to -0023976_125742_S1_M5_c4591001-A-report-cci-pulmonary.xlsx
FDA-CBER-2021-5683-0023979 to -0023980_125742_S1_M5_c4591001-A-report-cci-renal.xlsx
FDA-CBER-2021-5683-0023983 to -0023984_125742_S1_M5_c4591001-A-report-cci-rheumatic.xlsx
FDA-CBER-2021-5683-0023987_125742_S1_M5_c4591001-A-201114-hiv-preferred-terms.xlsx
pd-production-032422
125742_S1_M1_priority-review-request.pdf
pd-production-040122
125742_S1_M5_5351_c4591001-fa-interim-audit-certificates.pdf
125742_S1_M5_5351_c4591001-fa-interim-demographics.pdf
125742_S1_M5_5351_c4591001-fa-interim-iec-irb-consent-form.pdf
125742_S1_M5_5351_c4591001-fa-interim-invest-signature.pdf
125742_S1_M5_5351_c4591001-fa-interim-protocol-deviations.pdf
125742_S1_M5_5351_c4591001-fa-interim-publications.pdf
125742_S1_M5_5351_c4591001-fa-interim-randomization-sensitive.pdf
125742_S1_M5_5351_c4591001-fa-interim-randomization.pdf
125742_S1_M5_5351_c4591001-fa-interim-sample-crf.pdf
125742_S1_M5_5351_c4591001-fa-interim-sponsor-signature.pdf
125742_S1_M5_5351_c4591001-interim-mth6-adverse-events-sensitive.pdf
125742_S1_M5_5351_c4591001-interim-mth6-audit-certificates.pdf
125742_S1_M5_5351_c4591001-interim-mth6-demographics.pdf
125742_S1_M5_5351_c4591001-interim-mth6-invest-signature.pdf
reissue_5.3.6 postmarketing experience.pdf
pd-production-050222
125742_S1_M5_5351_bnt162-01-interim3-adverse-events.pdf
125742_S1_M5_5351_bnt162-01-interim3-compliance.pdf
125742_S1_M5_5351_bnt162-01-interim3-demographics.pdf
125742_S1_M5_5351_bnt162-01-interim3-discontinued-patients.pdf
125742_S1_M5_5351_bnt162-01-interim3-excluded-patients.pdf
125742_S1_M5_5351_bnt162-01-interim3-iec-irb.pdf
125742_S1_M5_5351_bnt162-01-interim3-invest-signature.pdf
125742_S1_M5_5351_bnt162-01-interim3-investigators.pdf
125742_S1_M5_5351_bnt162-01-interim3-lab-measurements.pdf
125742_S1_M5_5351_bnt162-01-interim3-notes-for-reader.pdf
125742_S1_M5_5351_bnt162-01-interim3-patient-batches.pdf
125742_S1_M5_5351_bnt162-01-interim3-protocol-deviations.pdf
125742_S1_M5_5351_bnt162-01-interim3-protocol.pdf
125742_S1_M5_5351_bnt162-01-interim3-report-body.pdf
125742_S1_M5_5351_bnt162-01-interim3-sample-crf.pdf
125742_S1_M5_5351_bnt162-01-interim3-sap.pdf
125742_S1_M5_5351_bnt162-01-interim3-sponsor-personnel-list.pdf
125742_S1_M5_5351_bnt162-01-interim3-sponsor-signature.pdf
125742_S1_M5_5351_bnt162-01-interim3-synopsis.pdf
125742_S1_M5_5351_bnt162-01_10010.pdf
125742_S1_M5_5351_bnt162-01_10075.pdf
125742_S1_M5_5351_bnt162-01_20116.pdf
125742_S1_M5_5351_bnt162-01_20215.pdf
125742_S1_M5_5351_bnt162-01_20242.pdf
125742_S1_M5_5351_c4591001-interim-mth6-randomization-sensitive.pdf
125742_S1_M5_5351_c4591001-interim-mth6-sample-crf.pdf
125742_S1_M5_5351_c4591001-interim-mth6-sponsor-signature.pdf
125742_S1_M5_bnt162-01-A-adrg.pdf
125742_S1_M5_bnt162-01-S-acrf.pdf
125742_S1_M5_bnt162-01-S-csdrg.pdf
125742_S1_M5_c4591001-S-acrf.pdf
125742_S1_M5_c4591001-S-csdrg.pdf
125742_S1_M5_c4591001-S-Supp-acrf.pdf
125742_S1_M5_CRF_c4591001-1001-10011093.pdf
125742_S1_M5_CRF_c4591001-1001-10011100.pdf
125742_S1_M5_CRF_c4591001-1001-10011135.pdf
125742_S1_M5_CRF_c4591001-1003-10031038.pdf
125742_S1_M5_CRF_c4591001-1003-10031065.pdf
125742_S1_M5_CRF_c4591001-1003-10031111.pdf
125742_S1_M5_CRF_c4591001-1003-10031113.pdf
125742_S1_M5_CRF_c4591001-1003-10031149.pdf
125742_S1_M5_CRF_c4591001-1003-10031186.pdf
125742_S1_M5_CRF_c4591001-1003-10031197.pdf
125742_S1_M5_CRF_c4591001-1003-10031207.pdf
125742_S1_M5_CRF_c4591001-1005-10051047.pdf
125742_S1_M5_CRF_c4591001-1005-10051054.pdf
125742_S1_M5_CRF_c4591001-1005-10051069.pdf
125742_S1_M5_CRF_c4591001-1005-10051214.pdf
125742_S1_M5_CRF_c4591001-1005-10051293.pdf
125742_S1_M5_CRF_c4591001-1005-10051347.pdf
125742_S1_M5_CRF_c4591001-1005-10051387.pdf
125742_S1_M5_CRF_c4591001-1005-10051411.pdf
125742_S1_M5_CRF_c4591001-1006-10061020.pdf
125742_S1_M5_CRF_c4591001-1006-10061040.pdf
125742_S1_M5_CRF_c4591001-1006-10061052.pdf
125742_S1_M5_CRF_c4591001-1006-10061094.pdf
125742_S1_M5_CRF_c4591001-1006-10061098.pdf
125742_S1_M5_CRF_c4591001-1006-10061176.pdf
FDA-CBER-2021-5683-0058316 to -0058458_125742_S1_M5_c4591001-A-Supp-define.xml
FDA-CBER-2021-5683-0058459 to -0058478_125742_S1_M5_c4591001-A-Supp-define-2-0-0.xsl
FDA-CBER-2021-5683-0058479 to -0058594_125742_S1_M5_c4591001-S-define.xml
FDA-CBER-2021-5683-0058595 to -0058614_125742_S1_M5_c4591001-S-define-2-0-0.xsl
FDA-CBER-2021-5683-0058615 to -0058652_125742_S1_M5_c4591001-S-Supp-define.xml
FDA-CBER-2021-5683-0058653 to -0058675_125742_S1_M5_c4591001-S-Supp-define-2-0-0.xsl
FDA-CBER-2021-5683-0058676 to -0058794_125742_S1_M5_bnt162-01-A-define.xml
FDA-CBER-2021-5683-0058795 to -0058828_125742_S1_M5_bnt162-01-A-define-2-0-0.xsl
FDA-CBER-2021-5683-0058829 to -0058954_125742_S1_M5_bnt162-01-S-define.xml
FDA-CBER-2021-5683-0058955 to -0058999_125742_S1_M5_bnt162-01-S-define-2-0-0.xsl
FDA-CBER-2021-5683-0059000 to -0065773_125742_S1_M5_c4591001-A-D-adcevd.xpt
FDA-CBER-2021-5683-0065774 to -0066700_125742_S1_M5_c4591001-A-D-addv.xpt
FDA-CBER-2021-5683-0066701 to -0123167_125742_S1_M5_c4591001-A-D-adfacevd.xpt
FDA-CBER-2021-5683-0123168 to -0126026_125742_S1_M5_c4591001-A-D-adva.xpt
pd-production-060122
125742_S1_M2_summary-biopharm.pdf
125742_S1_M5_5314_shi-sop-10011.pdf
125742_S1_M5_5351_c4591001-fa-interim-adverse-events.pdf
125742_S1_M5_5351_c4591001-fa-interim-discontinued-patients.pdf
125742_S1_M5_5351_c4591001-fa-interim-patient-batches.pdf
125742_S1_M5_5351_c4591001-fa-interim-protocol-deviations-sensitive.pdf
125742_S1_M5_5351_c4591001-interim-mth6-patient-batches.pdf
125742_S1_M5_5351_c4591001-interim-mth6-publications.pdf
125742_S1_M5_5351_c4591001-interim-mth6-randomization.pdf
125742_S1_M5_CRF_c4591001-1007-10071050.pdf
125742_S1_M5_CRF_c4591001-1007-10071097.pdf
125742_S1_M5_CRF_c4591001-1007-10071101.pdf
125742_S1_M5_CRF_c4591001-1007-10071117.pdf
125742_S1_M5_CRF_c4591001-1007-10071124.pdf
125742_S1_M5_CRF_c4591001-1007-10071159.pdf
125742_S1_M5_CRF_c4591001-1007-10071192.pdf
125742_S1_M5_CRF_c4591001-1007-10071276.pdf
125742_S1_M5_CRF_c4591001-1007-10071280.pdf
125742_S1_M5_CRF_c4591001-1007-10071306.pdf
125742_S1_M5_CRF_c4591001-1007-10071315.pdf
125742_S1_M5_CRF_c4591001-1007-10071347.pdf
125742_S1_M5_CRF_c4591001-1007-10071441.pdf
125742_S1_M5_CRF_c4591001-1007-10071443.pdf
125742_S1_M5_CRF_c4591001-1008-10081056.pdf
125742_S1_M5_CRF_c4591001-1008-10081152.pdf
125742_S1_M5_CRF_c4591001-1008-10081184.pdf
125742_S1_M5_CRF_c4591001-1008-10081337.pdf
125742_S1_M5_CRF_c4591001-1008-10081603.pdf
125742_S1_M5_CRF_c4591001-1008-10081628.pdf
125742_S1_M5_CRF_c4591001-1008-10081667.pdf
125742_S1_M5_CRF_c4591001-1009-10091123.pdf
125742_S1_M5_CRF_c4591001-1009-10091128.pdf
125742_S1_M5_CRF_c4591001-1009-10091135.pdf
125742_S1_M5_CRF_c4591001-1009-10091149.pdf
125742_S1_M5_CRF_c4591001-1011-10111029.pdf
125742_S1_M5_CRF_c4591001-1011-10111181.pdf
125742_S1_M5_CRF_c4591001-1012-10121097.pdf
125742_S1_M5_CRF_c4591001-1012-10121112.pdf
125742_S1_M5_CRF_c4591001-1012-10121163.pdf
125742_S1_M5_CRF_c4591001-1013-10131084.pdf
125742_S1_M5_CRF_c4591001-1013-10131089.pdf
125742_S1_M5_CRF_c4591001-1013-10131165.pdf
125742_S1_M5_CRF_c4591001-1013-10131176.pdf
125742_S1_M5_CRF_c4591001-1013-10131190.pdf
125742_S1_M5_CRF_c4591001-1013-10131229.pdf
125742_S1_M5_CRF_c4591001-1013-10131255.pdf
125742_S1_M5_CRF_c4591001-1013-10131386.pdf
125742_S1_M5_CRF_c4591001-1013-10131517.pdf
125742_S1_M5_CRF_c4591001-1013-10131554.pdf
125742_S1_M5_CRF_c4591001-1013-10131653.pdf
125742_S1_M5_CRF_c4591001-1013-10131656.pdf
125742_S1_M5_CRF_c4591001-1013-10131658.pdf
125742_S1_M5_CRF_c4591001-1013-10131699.pdf
125742_S1_M5_CRF_c4591001-1013-10131718.pdf
125742_S1_M5_CRF_c4591001-1013-10131786.pdf
125742_S1_M5_CRF_c4591001-1015-10151011.pdf
125742_S1_M5_CRF_c4591001-1015-10151035.pdf
125742_S1_M5_CRF_c4591001-1015-10151047.pdf
125742_S1_M5_CRF_c4591001-1015-10151071.pdf
125742_S1_M5_CRF_c4591001-1015-10151089.pdf
125742_S1_M5_CRF_c4591001-1015-10151101.pdf
125742_S1_M5_CRF_c4591001-1015-10151134.pdf
125742_S1_M5_CRF_c4591001-1015-10151225.pdf
125742_S1_M5_CRF_c4591001-1015-10151238.pdf
125742_S1_M5_CRF_c4591001-1081-10811194 reissue.pdf
125742_S1_M5_CRF_c4591001-1128-11281009 reissue.pdf
FDA-CBER-2021-5683-0142307 to -0149081_125742_S1_M5_c4591001-A-Supp-D-adcevd.xpt
FDA-CBER-2021-5683-0149082 to -0158559_125742_S1_M5_c4591001-S-D-ce.xpt
FDA-CBER-2021-5683-0158560 to -0159486_125742_S1_M5_c4591001-S-D-dv.xpt
FDA-CBER-2021-5683-0159487 to -0162689_125742_S1_M5_c4591001-S-D-ec.xpt
FDA-CBER-2021-5683-0162690 to -0165892_125742_S1_M5_c4591001-S-D-ex.xpt
FDA-CBER-2021-5683-0168683 to -0169081_125742_S1_M5_c4591001-S-D-suppcm.xpt
FDA-CBER-2021-5683-0169082 to -0171523_125742_S1_M5_c4591001-S-D-suppdm.xpt
FDA-CBER-2021-5683-0171524 to -0174606_125742_S1_M5_c4591001-S-D-suppds.xpt
FDA-CBER-2021-5683-0174607 to -0178318_125742_S1_M5_c4591001-S-D-suppdv.xpt
FDA-CBER-2021-5683-0178319 to -0180190_125742_S1_M5_c4591001-S-D-suppho.xpt
FDA-CBER-2021-5683-0180191 to -0182980_125742_S1_M5_c4591001-S-D-suppis.xpt
FDA-CBER-2021-5683-0182981 to -0187905_125742_S1_M5_c4591001-S-D-suppmh.xpt
FDA-CBER-2021-5683-0187906 to -0196048_125742_S1_M5_c4591001-S-D-suppvs.xpt
FDA-CBER-2021-5683-0196049 to -0205317_125742_S1_M5_c4591001-S-Supp-D-ce.xpt
FDA-CBER-2021-5683-0205318 to -0208520_125742_S1_M5_c4591001-S-Supp-D-ex.xpt
FDA-CBER-2021-5683-0208521 to -0216666_125742_S1_M5_c4591001-S-Supp-D-suppvs.xpt
pd-production-070122
125742_S1_M5_5351_c4591001-fa-interim-narrative-sensitive.pdf
125742_S1_M5_5351_c4591001-fa-interim-sap.pdf
125742_S1_M5_5351_c4591001-interim-mth6-adverse-events.pdf
125742_S1_M5_5351_c4591001-interim-mth6-compliance-sensitive.pdf
125742_S1_M5_5351_c4591001-interim-mth6-discontinued-patients.pdf
125742_S1_M5_5351_c4591001-interim-mth6-efficacy-response.pdf
125742_S1_M5_5351_c4591001-interim-mth6-errata.pdf
125742_S1_M5_5351_c4591001-interim-mth6-excluded-patients-sensitive.pdf
125742_S1_M5_5351_c4591001-interim-mth6-excluded-patients.pdf
125742_S1_M5_5351_c4591001-interim-mth6-interlab-standard.pdf
125742_S1_M5_5351_c4591001-interim-mth6-lab-measurements-sensitive.pdf
125742_S1_M5_5351_c4591001-interim-mth6-protocol-deviations.pdf
125742_S1_M5_5351_c4591001-interim-mth6-sap.pdf
125742_S2_M1_com195lkz-carton-kzoo.pdf
125742_S2_M1_com195lpus-carton-puurs.pdf
125742_S2_M1_com25ctkz-carton-kzoo.pdf
125742_S2_M1_com25ctpus-carton-puurs.pdf
125742_S2_M1_comvlabkz-vial-kzoo.pdf
125742_S2_M1_comvlabp-vial-puurs.pdf
125742_S2_M1_fk-diluent-carton.pdf
125742_S2_M1_fk-diluent-stamp.pdf
125742_S2_M1_hospira-diluent-carton.pdf
125742_S2_M1_hospira-diluent-label.pdf
125742_S2_M1_loa-dmf-011321-vials.pdf
125742_S2_M1_loa-dmf-011793-vials.pdf
125742_S2_M1_loa-dmf-011820-vials.pdf
125742_S2_M1_loa-dmf-012683-vials.pdf
125742_S2_M1_loa-dmf-031786-vials.pdf
125742_S2_M1_loa-dmf-10953-stopper.pdf
125742_S2_M1_right-of-reference.pdf
125742_S2_M5_5354_wi235284-protocol.pdf
FDA-CBER-2021-5683-0225100 to -0225597_125742_S1_M5_c4591001-A-D-adcm.xpt
FDA-CBER-2021-5683-0225598 to -0282129_125742_S1_M5_c4591001-A-Supp-D-adfacevd.xpt
FDA-CBER-2021-5683-0282130 to -0282328_125742_S1_M5_c4591001-S-D-cm.xpt
FDA-CBER-2021-5683-0282329 to -0282365_125742_S1_M5_c4591001-S-D-ie.xpt
FDA-CBER-2021-5683-0282366 to -0285643_125742_S1_M5_c4591001-S-D-mb.xpt
FDA-CBER-2021-5683-0285644 to -0285651_125742_S1_M5_c4591001-S-D-mo.xpt
FDA-CBER-2021-5683-0285652 to -0286071_125742_S1_M5_c4591001-S-D-pe.xpt
FDA-CBER-2021-5683-0286072 to -0286266_125742_S1_M5_c4591001-S-Supp-D-relrec.xpt
FDA-CBER-2021-5683-0286267 to -0296903_125742_S1_M5_c4591001-S-Supp-D-suppce.xpt
FDA-CBER-2021-5683-0296904 to -0307543_125742_S1_M5_c4591001-S-D-suppce.xpt
pd-production-080122
125742_S2_M5_54_ezeanolue-e-2019.pdf
125742_S2_3.PSUR-1.pdf
125742_S2_3_pfizer-report
FDA-CBER-2021-5683-0396187_125742_S1_M5_c4591001-S-D-pr.xpt
FDA-CBER-2021-5683-0396188 to -0396192_125742_S1_M5_c4591001-S-D-suppmo.xpt
FDA-CBER-2021-5683-0396193 to -0396194_125742_S1_M5_c4591001-S-D-supppe.xpt
FDA-CBER-2021-5683-0396195_125742_S1_M5_bnt162-01-S-D-relrec.xpt
FDA-CBER-2021-5683-0396196_125742_S1_M5_bnt162-01-S-D-supppe.xpt
FDA-CBER-2021-5683-0396197_125742_S1_M5_bnt162-01-S-D-supprp.xpt
FDA-CBER-2021-5683-0396198_125742_S1_M5_bnt162-01-S-D-te.xpt
FDA-CBER-2021-5683-0396199_125742_S1_M5_bnt162-01-S-D-tv.xpt
pd-production-010323
106A_2_BLA_125742-0_08-20-2021_Telecon_Labeling via FAX_e.pdf
10_BLA_125742-0_06-29-2021_Filing_Meeting_Summary.pdf
114A_2_BLA_125742-0_08-21-2021_Telecon_Labeling via FAX_e.pdf
125742_S12_M1_13jul2021-response.pdf
125742_S12_M1_waiver-req-preservative.pdf
125742_S13_M5_5351_c4591001-adae-hiv-listing.pdf
125742_S13_M5_5351_c4591001-narrative-1226-12262255.pdf
125742_S16_M1_ped-studies-deferral-track-change.pdf
125742_S16_M1_psp-track.pdf
125742_S1_M5_5351_c4591001-interim-mth6-iec-irb-consent-form.pdf
125742_S21_M1_pharmacovigilance-plan.pdf
125742_S21_M1_pvp-track.pdf
125742_S27_M1_response-29jul2021.pdf
125742_S28_M1_response-22jul2021-followup.pdf
125742_S29_M1_lab-1448-0-2-annotated.pdf
125742_S29_M1_lab-1448-0-2-pkg-insert-clean.pdf
125742_S29_M1_lab-1448-0-2-pkg-insert-track.pdf
125742_S33_M5_5351_c4591001-508-efficacy-tables.pdf
125742_S38_M5_c4591001-508-safety-tables.pdf
125742_S39_M1_5351_c4591001-ve-tables.pdf
125742_S39_M1_lab-1448-0-3-annotated.pdf
125742_S39_M1_lab-1448-0-3-pkg-insert-clean.pdf
125742_S39_M1_lab-1448-0-3-pkg-insert-track.pdf
125742_S41_M1_c4591001-ve-tables.pdf
125742_S46_M1_carton-com195lkz-kzoo.pdf
125742_S46_M1_carton-com195lpus-puurs.pdf
125742_S46_M1_carton-com25ctkz-kzoo.pdf
125742_S46_M1_carton-com25ctpus-puurs.pdf
125742_S46_M1_label-comvlabkz-kzoo.pdf
125742_S46_M1_label-comvlabp-puurs.pdf
125742_S46_M1_sticker-comdilrm.pdf
125742_S51_M1_lab-1448-0-4-annotated.pdf
125742_S51_M1_lab-1448-0-4-pkg-insert-clean.pdf
125742_S51_M1_lab-1448-0-4-pkg-insert-track.pdf
125742_S51_M5_5351_c4591001-followup-table.pdf
125742_S54_M1_carton-com195lkz-kzoo.pdf
125742_S54_M1_carton-com195lpus-puurs.pdf
125742_S54_M1_carton-com25ctkz-kzoo.pdf
125742_S54_M1_carton-com25ctpus-puurs.pdf
125742_S58_M1_lab-1448-0-5-annotated.pdf
125742_S58_M1_lab-1448-0-5-pkg-insert-clean.pdf
125742_S58_M1_lab-1448-0-5-pkg-insert-track.pdf
125742_S64_M1_carton-com195lkz-kzoo.pdf
125742_S64_M1_carton-com195lpus-puurs.pdf
125742_S64_M1_carton-com25ctkz-kzoo.pdf
125742_S64_M1_carton-com25ctpus-puurs.pdf
125742_S64_M1_label-comvlabkz-kzoo.pdf
125742_S64_M1_label-comvlabp-puurs.pdf
125742_S64_M1_sticker-comdilrm.pdf
125742_S68_M1_lab-1448-0-6-annotated.pdf
125742_S68_M1_lab-1448-0-6-pkg-insert-clean.pdf
125742_S68_M1_lab-1448-0-6-pkg-insert-track.pdf
125742_S6_M5_5351_c4591001-sequencing-listings.pdf
125742_S70_M1_lab-1448-0-7-annotated.pdf
125742_S70_M1_lab-1448-0-7-pkg-insert-clean.pdf
125742_S70_M1_lab-1448-0-7-pkg-insert-track.pdf
125742_S73_M1_lab-1448-0-8-annotated.pdf
125742_S73_M1_lab-1448-0-8-pkg-insert-clean.pdf
125742_S73_M1_lab-1448-0-8-pkg-insert-track.pdf
125742_S76_M1_lab-1448-0-9-annotated.pdf
125742_S76_M1_lab-1448-0-9-pkg-insert-clean.pdf
125742_S76_M1_lab-1448-0-9-pkg-insert-track.pdf
125742_S79_M1_lab-1448-1-0-pkg-insert-clean.pdf
125742_S7_M1_response-08jun2021.pdf
125742_S80_M1_lab-1448-1-0-pkg-insert-clean.pdf
126_BLA 125742-0_08-23-2021_Memo_Other.pdf
130_BLA _125742-0_09-01-2021_Committee Memo_Labeling.pdf
13_BLA_125742-0_07-02-2021_Committee Memo_APLB.pdf
36A_BLA_125742-0_07-28-2021_Telecon_Labeling Target Cl.pdf
4_BLA_125742-0_06-03-2021_First_Committee_Meeting_Summary.pdf
72A_BLA_125742-0_08-13-2021_Telecon_Labeling via FAX_e.pdf
77_BLA_125742-0_08-17-2021_Memo_Lot Release Clearance.pdf
78_BLA_125742-0_08-17-2021_Memo_Other.pdf
7_BLA_125742-0_06-17-2021_Memo_Other.pdf
82A_BLA_125742-0_08-17-2021_Telecon_Labeling via FAX_e.pdf
91A_2_BLA_125742-0_08-18-2021_Telecon_Labeling via FAX_e.pdf
98A_BLA_125742-0_08-19-2021_Telecon_Labeling via FAX_e.pdf
FDA-CBER-2021-5683-0652385-0652413-125742_S29_M1_lab-1448-0-2-pkg-insert-clean.docx
FDA-CBER-2021-5683-0652414-0652442-125742_S29_M1_lab-1448-0-2-pkg-insert-track.doc
FDA-CBER-2021-5683-0652443-0652471-125742_S33_M5_5351_c4591001-508-efficacy-tables.doc
FDA-CBER-2021-5683-0652472-0652503-125742_S38_M5_c4591001-508-safety-tables.doc.docx
FDA-CBER-2021-5683-0652504-0652525-125742_S39_M1_lab-1448-0-3-pkg-insert-track.doc
FDA-CBER-2021-5683-0652526-0652547-125742_S39_M1_lab-1448-0-3-annotated.doc
FDA-CBER-2021-5683-0652548-0652569-125742_S39_M1_lab-1448-0-3-pkg-insert-clean.doc
FDA-CBER-2021-5683-0652570-0652590-125742_S51_M1_lab-1448-0-4-pkg-insert-track.docx
FDA-CBER-2021-5683-0652591-0652611-125742_S51_M1_lab-1448-0-4-annotated.docx
FDA-CBER-2021-5683-0652612-0652632-125742_S51_M1_lab-1448-0-4-pkg-insert-clean.docx
FDA-CBER-2021-5683-0652633-0652652-125742_S58_M1_lab-1448-0-5-annotated.docx
FDA-CBER-2021-5683-0652653-0652672-125742_S58_M1_lab-1448-0-5-pkg-insert-track.docx
FDA-CBER-2021-5683-0652673-0652692-125742_S58_M1_lab-1448-0-5-pkg-insert-clean.docx
FDA-CBER-2021-5683-0652693-0652713-125742_S68_M1_lab-1448-0-6-annotated.docx
FDA-CBER-2021-5683-0652714-0652734-125742_S68_M1_lab-1448-0-6-pkg-insert-clean.docx
FDA-CBER-2021-5683-0652735-0652755-125742_S68_M1_lab-1448-0-6-pkg-insert-track.docx
FDA-CBER-2021-5683-0652756-0652776-125742_S70_M1_lab-1448-0-7-annotated.docx
FDA-CBER-2021-5683-0652777-0652797-125742_S70_M1_lab-1448-0-7-pkg-insert-clean.docx
FDA-CBER-2021-5683-0652798-0652818-125742_S70_M1_lab-1448-0-7-pkg-insert-track.docx
FDA-CBER-2021-5683-0652819-0652838-125742_S73_M1_lab-1448-0-8-pkg-insert-clean.docx
FDA-CBER-2021-5683-0652839-0652858-125742_S73_M1_lab-1448-0-8-annotated.docx
FDA-CBER-2021-5683-0652859-0652878-125742_S73_M1_lab-1448-0-8-pkg-insert-track.docx
FDA-CBER-2021-5683-0652879-0652898-125742_S76_M1_lab-1448-0-9-pkg-insert-clean.docx
FDA-CBER-2021-5683-0652899-0652919-125742_S76_M1_lab-1448-0-9-pkg-insert-track.docx
FDA-CBER-2021-5683-0652920-0652940-125742_S76_M1_lab-1448-0-9-annotated.docx
FDA-CBER-2021-5683-0652941-0652960-125742_S79_M1_lab-1448-1-0-pkg-insert-clean.docx
FDA-CBER-2021-5683-0652961-0652980-125742_S80_M1_lab-1448-1-0-pkg-insert-clean.docx
pd-production-020123
125742_S1_M2_2.6.1 introduction.pdf125742_S1_M2_2.6.2 pharmacol-written-summary.pdf
125742_S1_M2_2.6.3.1 pharmacol-tabulated-summary.pdf
125742_S1_M2_2.6.6 toxicology-written-summary.pdf
125742_S1_M2_2.6.7.1toxicology-tabulated-summary.pdf
125742_S1_M4_4.2.1 r-20-0085.pdf
125742_S1_M4_4.2.1 r-20-0112.pdf
125742_S1_M4_4.2.1 r-20-0211.pdf
125742_S1_M4_4.2.1 vr-vtr-10741.pdf
125742_S1_M4_4.2.2 072424.pdf
125742_S1_M4_4.2.2.1 vr-vtr-10671.pdf
125742_S1_M4_4.2.2.4 01049-20008.pdf
125742_S1_M4_4.2.2.4 01049-20009.pdf
125742_S1_M4_4.2.2.4 01049-20010.pdf
125742_S1_M4_4.2.2.4 01049-20020.pdf
125742_S1_M4_4.2.2.4 01049-20021.pdf
125742_S1_M4_4.2.2.4 01049-20022.pdf
125742_S1_M4_4.2.2.4 043725.pdf
125742_S1_M4_4.2.3.2 20gr142.pdf
125742_S1_M4_4.3 munster-jv-2020.pdf
pd-production-030123
125742_S1_M4_20gr142_nsdrg.pdf125742_S1_M4_38166_nsdrg.pdf
125742_S1_M4_4.2.3.2 38166.pdf
FDA-CBER-2021-5683-0767981-0768062_125742_S1_M4_20gr142_define.xml
FDA-CBER-2021-5683-0768063-0768092_125742_S1_M4_20gr142_define2-0-0.xsl
FDA-CBER-2021-5683-0768093-0768158_125742_S1_M4_38166_define.xml
FDA-CBER-2021-5683-0768159-0768188_125742_S1_M4_38166_define2-0-0.xsl
pd-production-040323
125742_S14_M1_cover.pdf125742_S1_M5_5314_vr-mqr-10211-att-01.pdf
125742_S1_M5_5314_vr-mqr-10211.pdf
125742_S1_M5_5314_vr-mqr-10212-att-01.pdf
125742_S1_M5_5314_vr-mqr-10212.pdf
125742_S1_M5_5314_vr-mqr-10214-att-01.pdf
125742_S1_M5_5314_vr-mqr-10214-att-02.pdf
125742_S1_M5_5314_vr-mqr-10214-att-03.pdf
125742_S1_M5_5314_vr-mqr-10214.pdf
125742_S1_M5_5314_vr-mvr-10080-att-01.pdf
125742_S1_M5_5314_vr-mvr-10080-att-02.pdf
125742_S1_M5_5314_vr-mvr-10080.pdf
125742_S1_M5_5314_vr-mvr-10081-att-01.pdf
125742_S1_M5_5314_vr-mvr-10081.pdf
125742_S1_M5_5314_vr-mvr-10083-att01.pdf
125742_S1_M5_5314_vr-mvr-10083-att02.pdf
125742_S1_M5_5314_vr-mvr-10083-att03.pdf
125742_S1_M5_5314_vr-mvr-10083-att04.pdf
125742_S1_M5_5314_vr-mvr-10083.pdf
125742_S1_M5_5314_vr-sop-lc-11120.pdf
125742_S1_M5_5314_vr-tm-10293.pdf
125742_S1_M5_5314_vr-tm-10294.pdf
125742_S1_M5_5314_vr-tm-10298.pdf
125742_S1_M5_5314_vr-tm-10304.pdf
125742_S1_M5_5351_c4591001-fa-interim-report-body.pdf
125742_S1_M5_5351_c4591001-fa-interim-synopsis.pdf
125742_S1_M5_5351_c4591001-interim-mth6-report-body.pdf
125742_S1_M5_5351_c4591001-interim-mth6-synopsis.pdf
125742_S2_M1_cover.pdf
125742_S2_M1_dca-anaphylactic-reaction.pdf
125742_S2_M1_dca-vaed.pdf
125742_S2_M1_diluent-labeling-proposal.pdf
125742_S2_M1_loa-dmf-9543-vials.pdf
125742_S2_M1_pllr-cumulative-review.pdf
125742_S2_M1_pllr-lit-search-summary.pdf
125742_S2_M1_pllr-waiver-request-drug-utilization.pdf
125742_S2_M1_pllr-waiver-request-pregnancy-registry.pdf
125742_S2_M5_5351_c4591007-protocol-amend1.pdf
125742_S2_M5_5351_c4591015-protocol-amend2.pdf
125742_S2_M5_5354_c4591008-protocol.pdf
125742_S2_M5_5354_c4591009-protocol-synopsis.pdf
125742_S2_M5_5354_c4591011-protocol.pdf
125742_S2_M5_5354_c4591012-protocol.pdf
125742_S2_M5_5354_c4591014-protocol.pdf
125742_S2_M5_5354_c4591022-protocol.pdf
125742_S2_M5_5354_wi255886-protocol.pdf
125742_S3_M1_cover.pdf
125742_S3_M1_proprietary-name.pdf
125742_S4_M1_cover.pdf
125742_S4_M1_response-18-may-2021-datasets.pdf
125742_S7_M5_5351_bnt162-01-interim3-reports.pdf
pd-production-050123
125742_S1_M5_CRF_c4591001-1016-10161042.pdf125742_S1_M5_CRF_c4591001-1016-10161087.pdf
125742_S1_M5_CRF_c4591001-1016-10161103.pdf
125742_S1_M5_CRF_c4591001-1016-10161120.pdf
125742_S1_M5_CRF_c4591001-1016-10161128.pdf
125742_S1_M5_CRF_c4591001-1016-10161199.pdf
125742_S1_M5_CRF_c4591001-1016-10161265.pdf
125742_S1_M5_CRF_c4591001-1016-10161277.pdf
125742_S1_M5_CRF_c4591001-1016-10161289.pdf
125742_S1_M5_CRF_c4591001-1018-10181031.pdf
125742_S1_M5_CRF_c4591001-1018-10181090.pdf
125742_S1_M5_CRF_c4591001-1018-10181111.pdf
125742_S1_M5_CRF_c4591001-1018-10181132.pdf
125742_S1_M5_CRF_c4591001-1018-10181159.pdf
125742_S1_M5_CRF_c4591001-1036-10361140.pdf
125742_S1_M5_CRF_c4591001-1066-10661202.pdf
125742_S1_M5_CRF_c4591001-1066-10661242.pdf
125742_S1_M5_CRF_c4591001-1066-10661350.pdf
125742_S1_M5_CRF_c4591001-1091-10911002.pdf
125742_S1_M5_CRF_c4591001-1091-10911170.pdf
125742_S1_M5_CRF_c4591001-1091-10911197.pdf
125742_S1_M5_CRF_c4591001-1091-10911213.pdf
125742_S1_M5_CRF_c4591001-1091-10911247.pdf
125742_S1_M5_CRF_c4591001-1091-10911274.pdf
125742_S1_M5_CRF_c4591001-1091-10911297.pdf
125742_S1_M5_CRF_c4591001-1091-10911299.pdf
125742_S1_M5_CRF_c4591001-1091-10911300.pdf
125742_S1_M5_CRF_c4591001-1112-11121118.pdf
125742_S1_M5_CRF_c4591001-1112-11121122.pdf
125742_S1_M5_CRF_c4591001-1112-11121193.pdf
125742_S1_M5_CRF_c4591001-1112-11121255.pdf
125742_S1_M5_CRF_c4591001-1112-11121301.pdf
125742_S1_M5_CRF_c4591001-1112-11121337.pdf
125742_S1_M5_CRF_c4591001-1114-11141006.pdf
125742_S1_M5_CRF_c4591001-1114-11141050.pdf
125742_S1_M5_CRF_c4591001-1114-11141075.pdf
125742_S1_M5_CRF_c4591001-1114-11141080.pdf
125742_S1_M5_CRF_c4591001-1117-11171036.pdf
125742_S1_M5_CRF_c4591001-1117-11171058.pdf
125742_S1_M5_CRF_c4591001-1117-11171079.pdf
125742_S1_M5_CRF_c4591001-1117-11171086.pdf
125742_S1_M5_CRF_c4591001-1117-11171088.pdf
125742_S1_M5_CRF_c4591001-1117-11171121.pdf
125742_S1_M5_CRF_c4591001-1117-11171141.pdf
125742_S1_M5_CRF_c4591001-1117-11171146.pdf
125742_S1_M5_CRF_c4591001-1117-11171167.pdf
125742_S1_M5_CRF_c4591001-1117-11171186.pdf
125742_S1_M5_CRF_c4591001-1118-11181013.pdf
125742_S1_M5_CRF_c4591001-1118-11181031.pdf
125742_S1_M5_CRF_c4591001-1118-11181044.pdf
125742_S1_M5_CRF_c4591001-1118-11181057.pdf
125742_S1_M5_CRF_c4591001-1118-11181074.pdf
125742_S1_M5_CRF_c4591001-1118-11181123.pdf
125742_S1_M5_CRF_c4591001-1120-11201002.pdf
125742_S1_M5_CRF_c4591001-1120-11201050.pdf
125742_S1_M5_CRF_c4591001-1120-11201059.pdf
125742_S1_M5_CRF_c4591001-1120-11201127.pdf
125742_S1_M5_CRF_c4591001-1120-11201266.pdf
125742_S1_M5_CRF_c4591001-1120-11201350.pdf
125742_S1_M5_CRF_c4591001-1120-11201432.pdf
125742_S1_M5_CRF_c4591001-1121-11211112.pdf
125742_S1_M5_CRF_c4591001-1129-11291005.pdf
125742_S1_M5_CRF_c4591001-1129-11291032.pdf
125742_S1_M5_CRF_c4591001-1129-11291037.pdf
125742_S1_M5_CRF_c4591001-1129-11291045.pdf
125742_S1_M5_CRF_c4591001-1129-11291046.pdf
125742_S1_M5_CRF_c4591001-1129-11291059.pdf
125742_S1_M5_CRF_c4591001-1129-11291074.pdf
125742_S1_M5_CRF_c4591001-1129-11291095.pdf
125742_S1_M5_CRF_c4591001-1129-11291127.pdf
125742_S1_M5_CRF_c4591001-1129-11291138.pdf
125742_S1_M5_CRF_c4591001-1129-11291161.pdf
125742_S1_M5_CRF_c4591001-1129-11291166.pdf
125742_S1_M5_CRF_c4591001-1129-11291183.pdf
125742_S1_M5_CRF_c4591001-1129-11291260.pdf
125742_S1_M5_CRF_c4591001-1133-11331006.pdf
125742_S1_M5_CRF_c4591001-1133-11331147.pdf
125742_S1_M5_CRF_c4591001-1133-11331207.pdf
125742_S1_M5_CRF_c4591001-1133-11331317.pdf
125742_S1_M5_CRF_c4591001-1133-11331537.pdf
125742_S1_M5_CRF_c4591001-1133-11331640.pdf
125742_S1_M5_CRF_c4591001-1134-11341019.pdf
125742_S1_M5_CRF_c4591001-1134-11341058.pdf
125742_S1_M5_CRF_c4591001-1134-11341085.pdf
125742_S1_M5_CRF_c4591001-1134-11341153.pdf
125742_S1_M5_CRF_c4591001-1134-11341174.pdf
125742_S1_M5_CRF_c4591001-1134-11341250.pdf
125742_S1_M5_CRF_c4591001-1134-11341327.pdf
125742_S1_M5_CRF_c4591001-1134-11341378.pdf
125742_S1_M5_CRF_c4591001-1134-11341432.pdf
125742_S1_M5_CRF_c4591001-1145-11451042.pdf
125742_S1_M5_CRF_c4591001-1145-11451055.pdf
125742_S1_M5_CRF_c4591001-1145-11451056.pdf
125742_S1_M5_CRF_c4591001-1145-11451059.pdf
125742_S1_M5_CRF_c4591001-1145-11451063.pdf
125742_S1_M5_CRF_c4591001-1145-11451076.pdf
125742_S1_M5_CRF_c4591001-1146-11461015.pdf
125742_S1_M5_CRF_c4591001-1146-11461109.pdf
125742_S1_M5_CRF_c4591001-1146-11461133.pdf
125742_S1_M5_CRF_c4591001-1146-11461152.pdf
125742_S1_M5_CRF_c4591001-1146-11461161.pdf
125742_S1_M5_CRF_c4591001-1146-11461181.pdf
125742_S1_M5_CRF_c4591001-1146-11461191.pdf
125742_S1_M5_CRF_c4591001-1146-11461200.pdf
125742_S1_M5_CRF_c4591001-1146-11461235.pdf
125742_S1_M5_CRF_c4591001-1146-11461264.pdf
125742_S1_M5_CRF_c4591001-1146-11461302.pdf
125742_S1_M5_CRF_c4591001-1147-11471007.pdf
125742_S1_M5_CRF_c4591001-1147-11471230.pdf
125742_S1_M5_CRF_c4591001-1147-11471239.pdf
125742_S1_M5_CRF_c4591001-1149-11491239.pdf
125742_S1_M5_CRF_c4591001-1149-11491313.pdf
125742_S1_M5_CRF_c4591001-1150-11501001.pdf
125742_S1_M5_CRF_c4591001-1150-11501022.pdf
125742_S1_M5_CRF_c4591001-1150-11501037.pdf
125742_S1_M5_CRF_c4591001-1150-11501069.pdf
125742_S1_M5_CRF_c4591001-1150-11501084.pdf
125742_S1_M5_CRF_c4591001-1150-11501093.pdf
125742_S1_M5_CRF_c4591001-1150-11501153.pdf
125742_S1_M5_CRF_c4591001-1167-11671009.pdf
125742_S1_M5_CRF_c4591001-1167-11671069.pdf
125742_S1_M5_CRF_c4591001-1167-11671077.pdf
125742_S1_M5_CRF_c4591001-1167-11671085.pdf
125742_S1_M5_CRF_c4591001-1167-11671175.pdf
125742_S1_M5_CRF_c4591001-1168-11681083.pdf
125742_S1_M5_CRF_c4591001-1168-11681225.pdf
125742_S1_M5_CRF_c4591001-1169-11691010.pdf
125742_S1_M5_CRF_c4591001-1169-11691055.pdf
125742_S1_M5_CRF_c4591001-1169-11691056.pdf
125742_S1_M5_CRF_c4591001-1174-11741042.pdf
125742_S1_M5_CRF_c4591001-1178-11781012.pdf
125742_S1_M5_CRF_c4591001-1178-11781015.pdf
125742_S1_M5_CRF_c4591001-1178-11781025.pdf
125742_S1_M5_CRF_c4591001-1178-11781048.pdf
125742_S1_M5_CRF_c4591001-1178-11781061.pdf
125742_S1_M5_CRF_c4591001-1178-11781073.pdf
125742_S1_M5_CRF_c4591001-1178-11781107.pdf
125742_S1_M5_CRF_c4591001-1178-11781122.pdf
125742_S1_M5_CRF_c4591001-1178-11781138.pdf
125742_S1_M5_CRF_c4591001-1178-11781164.pdf
125742_S1_M5_CRF_c4591001-1178-11781167.pdf
125742_S1_M5_CRF_c4591001-1178-11781257.pdf
125742_S1_M5_CRF_c4591001-1178-11781287.pdf
125742_S1_M5_CRF_c4591001-1178-11781293.pdf
125742_S1_M5_CRF_c4591001-1178-11781300.pdf
125742_S1_M5_CRF_c4591001-1185-11851055.pdf
125742_S1_M5_CRF_c4591001-1194-11941002.pdf
125742_S1_M5_CRF_c4591001-1194-11941033.pdf
125742_S1_M5_CRF_c4591001-1194-11941058.pdf
125742_S1_M5_CRF_c4591001-1207-12071055.pdf
125742_S1_M5_CRF_c4591001-1213-12131049.pdf
125742_S1_M5_CRF_c4591001-1214-12141018.pdf
125742_S1_M5_CRF_c4591001-1214-12141039.pdf
125742_S1_M5_CRF_c4591001-1217-12171031.pdf
125742_S1_M5_CRF_c4591001-1217-12171039.pdf
125742_S1_M5_CRF_c4591001-1217-12171044.pdf
125742_S1_M5_CRF_c4591001-1218-12181001.pdf
125742_S1_M5_CRF_c4591001-1218-12181012.pdf
125742_S1_M5_CRF_c4591001-1218-12181015.pdf
125742_S1_M5_CRF_c4591001-1218-12181023.pdf
125742_S1_M5_CRF_c4591001-1218-12181046.pdf
125742_S1_M5_CRF_c4591001-1218-12181051.pdf
125742_S1_M5_CRF_c4591001-1218-12181057.pdf
125742_S1_M5_CRF_c4591001-1220-12201020.pdf
125742_S1_M5_CRF_c4591001-1220-12201044.pdf
125742_S1_M5_CRF_c4591001-1220-12201052.pdf
125742_S1_M5_CRF_c4591001-1221-12211002.pdf
125742_S1_M5_CRF_c4591001-1221-12211007.pdf
125742_S1_M5_CRF_c4591001-1223-12231014.pdf
125742_S1_M5_CRF_c4591001-1223-12231024.pdf
125742_S1_M5_CRF_c4591001-1223-12231058.pdf
125742_S1_M5_CRF_c4591001-1223-12231075.pdf
125742_S1_M5_CRF_c4591001-1223-12231159.pdf
125742_S1_M5_CRF_c4591001-1223-12231166.pdf
125742_S1_M5_CRF_c4591001-1223-12231181.pdf
125742_S1_M5_CRF_c4591001-1223-12231182.pdf
125742_S1_M5_CRF_c4591001-1223-12231252.pdf
125742_S1_M5_CRF_c4591001-1224-12241065.pdf
125742_S1_M5_CRF_c4591001-1229-12291083.pdf
125742_S1_M5_CRF_c4591001-1230-12301025.pdf
125742_S1_M5_CRF_c4591001-1230-12301045.pdf
125742_S1_M5_CRF_c4591001-1248-12481120.pdf
125742_S1_M5_CRF_c4591001-1248-12481163.pdf
125742_S1_M5_CRF_c4591001-1248-12481218.pdf
125742_S1_M5_CRF_c4591001-1251-12511029.pdf
125742_S1_M5_CRF_c4591001-1251-12511031.pdf
125742_S1_M5_CRF_c4591001-1251-12511033.pdf
125742_S1_M5_CRF_c4591001-1251-12511050.pdf
125742_S1_M5_CRF_c4591001-1251-12511060.pdf
125742_S1_M5_CRF_c4591001-1251-12511072.pdf
125742_S1_M5_CRF_c4591001-1251-12511145.pdf
125742_S1_M5_CRF_c4591001-1251-12511239.pdf
125742_S1_M5_CRF_c4591001-1251-12511250.pdf
125742_S1_M5_CRF_c4591001-1251-12511262.pdf
125742_S1_M5_CRF_c4591001-1252-12521010.pdf
125742_S1_M5_CRF_c4591001-1252-12521011.pdf
125742_S1_M5_CRF_c4591001-1254-12541006.pdf
125742_S1_M5_CRF_c4591001-1254-12541014.pdf
125742_S1_M5_CRF_c4591001-1254-12541109.pdf
125742_S1_M5_CRF_c4591001-1254-12541142.pdf
125742_S1_M5_CRF_c4591001-1254-12541145.pdf
125742_S1_M5_CRF_c4591001-1254-12541189.pdf
125742_S1_M5_CRF_c4591001-1260-12601018.pdf
125742_S1_M5_CRF_c4591001-1260-12601035.pdf
125742_S1_M5_CRF_c4591001-1260-12601037.pdf
125742_S1_M5_CRF_c4591001-1260-12601069.pdf
125742_S1_M5_CRF_c4591001-1260-12601075.pdf
125742_S1_M5_CRF_c4591001-1260-12601108.pdf
125742_S1_M5_CRF_c4591001-1260-12601128.pdf
125742_S1_M5_CRF_c4591001-1261-12611006.pdf
125742_S1_M5_CRF_c4591001-1264-12641133.pdf
125742_S1_M5_CRF_c4591001-1264-12641195.pdf
125742_S1_M5_CRF_c4591001-1264-12641229.pdf
125742_S1_M5_CRF_c4591001-1265-12651101.pdf
125742_S1_M5_CRF_c4591001-1265-12651149.pdf
125742_S1_M5_CRF_c4591001-1270-12701057.pdf
125742_S1_M5_CRF_c4591001-1270-12701069.pdf
125742_S1_M5_CRF_c4591001-1270-12701142.pdf
pd-production-100123
019736_S157_M1_1571.pdf019736_S157_M1_cover.pdf
019736_S157_M5_vr-mvp-10074.pdf
019736_S196_M1_1571.pdf
019736_S196_M1_cover.pdf
019736_S196_M5_c4591008-protocol.pdf
019736_S196_M5_c4591011-protocol.pdf
019736_S196_M5_c4591012-protocol.pdf
019736_S222_M1_1571.pdf
019736_S222_M1_cover.pdf
019736_S222_M5_c4591020-informed-consent-form.pdf
019736_S222_M5_c4591020-protocol.pdf
019736_S268_M1_1571.pdf
019736_S268_M1_cover.pdf
019736_S268_M5_c4591014-protocol.pdf
019736_S268_M5_wi235284-protocol.pdf
019736_S268_M5_wi255886-protocol.pdf
019736_S268_M5_wi255886-sap.pdf
019736_S296_M1_1571.pdf
019736_S31_M1_1571.pdf
019736_S31_M1_cover.pdf
019736_S31_M1_response-ir-06jul2020-c4591001.pdf
019736_S31_M5_vr-mqr-10211.pdf
019736_S31_M5_vr-mqr-10212.pdf
019736_S31_M5_vr-mqr-10214-att01-shi-sop-10011.pdf
019736_S31_M5_vr-mqr-10214.pdf
019736_S31_M5_vr-tm-10293.pdf
019736_S31_M5_vr-tm-10294.pdf
019736_S325_M1_1571.pdf
019736_S325_M1_cover.pdf
019736_S325_M5_c4591020-sap-track.pdf
019736_S325_M5_c4591020-sap.pdf
019736_S387_M1_1571.pdf
019736_S387_M1_cover.pdf
019736_S387_M5_c4591008-interim-abstract.pdf
019736_S387_M5_c4591008-interim-compliance.pdf
019736_S387_M5_c4591008-interim-demographics.pdf
019736_S387_M5_c4591008-interim-excluded-subjects.pdf
019736_S387_M5_c4591008-interim-non-hospitalization.pdf
019736_S387_M5_c4591008-interim-protocol-deviations.pdf
019736_S387_M5_c4591008-interim-protocol.pdf
019736_S387_M5_c4591008-interim-report-body.pdf
019736_S387_M5_c4591008-interim-sample-icd.pdf
019736_S387_M5_c4591008-interim-sap.pdf
019736_S387_M5_c4591008-interim-sponsor-signature.pdf
019736_S387_M5_c4591008-interim-unplanned-hospitalization.pdf
019736_S387_M5_c4591008-interim-withdrawn-subjects.pdf
019736_S387_M5_c4591012-interim-abstract.pdf
019736_S387_M5_c4591012-interim-protocol.pdf
019736_S387_M5_c4591012-interim-report-body.pdf
019736_S387_M5_c4591012-interim-sponsor-signature.pdf
019736_S398_M1_1571.pdf
019736_S398_M1_cover.pdf
019736_S398_M1_response-30june2021-icd-myo-peri.pdf
019736_S414_M1_1571.pdf
019736_S414_M1_cover.pdf
019736_S414_M5_c4591001-amendment-17-clean.pdf
019736_S414_M5_c4591001-amendment-17-track.pdf
019736_S427_M1_1571.pdf
019736_S444_M1_1571.pdf
019736_S444_M1_cover.pdf
019736_S444_M5_c4591007-p1-adult-participant-icd-lower-dose-eval.pdf
019736_S444_M5_c4591007-p1-older-children-assent-lower-dose-eval-icd.pdf
019736_S444_M5_c4591007-p1-parent-icd-lower-dose-eval.pdf
019736_S444_M5_c4591007-p1-younger-children-assent-lower-dose-eval-icd.pdf
019736_S444_M5_c4591007-p2-3-adult-participant-icd-lower-dose-eval.pdf
019736_S444_M5_c4591007-p2-3-children-assent--cont-select-dose-icd.pdf
019736_S444_M5_c4591007-p2-3-children-assent-pbo-cont-select-dose-icd-track.pdf
019736_S444_M5_c4591007-p2-3-older-child-assent-lower-dose-eval.pdf
019736_S444_M5_c4591007-p2-3-parent-icd-lower-dose-eval.pdf
019736_S444_M5_c4591007-p2-3-parent-icd-pbo-controlled-selected-dose-track.pdf
019736_S444_M5_c4591007-p2-3-parent-icd-pbo-controlled-selected-dose.pdf
019736_S444_M5_c4591007-p2-3-younger-child-assent-lower-dose-eval.pdf
019736_S444_M5_c4591007-protocol-amend2-track.pdf
019736_S444_M5_c4591007-protocol-amend2.pdf
019736_S470_M1_1571.pdf
019736_S470_M1_cover.pdf
019736_S470_M5_c4591009-protocol.pdf
019736_S470_M5_c4591012-protocol-track.pdf
019736_S470_M5_c4591012-protocol.pdf
019736_S488_M1_1571.pdf
019736_S488_M1_cover.pdf
019736_S488_M5_c4591007-p2-3-older-children-assent-troponin-icd.pdf
019736_S488_M5_c4591007-p2-3-parent-addendum-icd-pbo-cont-selected-dose.pdf
019736_S488_M5_c4591007-p2-3-parent-icd-pbo-controlled-selected-dose-track.pdf
019736_S488_M5_c4591007-p2-3-parent-icd-pbo-controlled-selected-dose.pdf
019736_S488_M5_c4591007-p2-3-parent-troponin-icd.pdf
019736_S488_M5_c4591007-p2-3-younger-children-assent-troponin-icd.pdf
019736_S488_M5_c4591007-protocol-amend3-track.pdf
019736_S488_M5_c4591007-protocol-amend3.pdf
019736_S70_M1_1571.pdf
019736_S70_M1_cover.pdf
019736_S70_M1_response-ir-29jul2020-clin-assay-qual.pdf
019736_S70_M5_vr-mqr-10211-10212-dlq.pdf
019736_S70_M5_vr-mqr-10211-10212-pq.pdf
019736_S70_M5_vr-mqr-10211-att01.pdf
019736_S70_M5_vr-mqr-10211.pdf
019736_S70_M5_vr-mqr-10212-att01.pdf
019736_S70_M5_vr-mqr-10212.pdf
019736_S70_M5_vr-mqr-10214-att01-shi-sop-10011.pdf
019736_S70_M5_vr-mqr-10214-att01.pdf
019736_S70_M5_vr-mqr-10214-att02.pdf
019736_S70_M5_vr-mqr-10214-att03.pdf
019736_S70_M5_vr-mqr-10214.pdf
019736_S70_M5_vr-sop-lc-11120.pdf
019736_S70_M5_vr-tm-10293.pdf
019736_S70_M5_vr-tm-10294.pdf
11_PM_S136_BLA 125742-31_10-30-2021_Memo_Final Review Memo.pdf
125742_2_BLA 125742-0_05-18-2021_Telecon_Information Reques.pdf
125742_S0136_M5_smsr-01sep2021-30sep2021.pdf
125742_S102_M1_356h.pdf
125742_S105_M1_356h.pdf
125742_S10_M1_356h.pdf
125742_S112_M1_356h.pdf
125742_S113_M1_356h.pdf
125742_S11_M1_356h.pdf
125742_S12_M1_356h.pdf
125742_S136_M1_356h.pdf
125742_S13_M1_356h.pdf
125742_S14_M1_356h.pdf
125742_S15_M1_356h.pdf
125742_S16_M1_356h.pdf
125742_S17_M1_356h.pdf
125742_S18_M1_356h.pdf
125742_S19_M1_356h.pdf
125742_S1_M1_meeting-correspondence.pdf
125742_S1_M5_5351_bnt162-01-interim3-reports.pdf
125742_S1_M5_5351_c4591001-fa-interim-interlab-standard.pdf
125742_S1_M5_5351_c4591001-fa-interim-investigators.pdf
125742_S1_M5_5351_c4591001-interim-mth6-investigators.pdf
125742_S20_M1_356h.pdf
125742_S21_M1_356h.pdf
125742_S22_M1_356h.pdf
125742_S23_M1_356h.pdf
125742_S24_M1_356h.pdf
125742_S25_M1_356h.pdf
125742_S26_M1_356h.pdf
125742_S27_M1_356h.pdf
125742_S28_M1_356h.pdf
125742_S29_M1_356h.pdf
125742_S2_M1_356h.pdf
125742_S2_M1_environmental-analysis.pdf
125742_S2_M5_5.4_sedic-m-2020.pdf
125742_S2_M5_5.4_singh-dk-2020.pdf
125742_S2_M5_5.4_who-2005.pdf
125742_S2_M5_5.4_who-2019.pdf
125742_S30_M1_356h.pdf
125742_S31_M1_356h.pdf
125742_S32_M1_356h.pdf
125742_S33_M1_356h.pdf
125742_S34_M1_356h.pdf
125742_S35_M1_356h.pdf
125742_S36_M1_356h.pdf
125742_S37_M1_356h.pdf
125742_S38_M1_356h.pdf
125742_S39_M1_356h.pdf
125742_S3_M1_356h.pdf
125742_S40_M1_356h.pdf
125742_S41_M1_356h.pdf
125742_S42_M1_356h.pdf
125742_S43_M1_356h.pdf
125742_S44_M1_356h.pdf
125742_S45_M1_356h.pdf
125742_S46_M1_356h.pdf
125742_S47_M1_356h.pdf
125742_S48_M1_356h.pdf
125742_S49_M1_356h.pdf
125742_S4_M1_356h.pdf
125742_S50_M1_356h.pdf
125742_S51_M1_356h.pdf
125742_S52_M1_356h.pdf
125742_S53_M1_356h.pdf
125742_S54_M1_356h.pdf
125742_S55_M1_356h.pdf
125742_S56_M1_356h.pdf
125742_S57_M1_356h.pdf
125742_S58_M1_356h.pdf
125742_S59_M1_356h.pdf
125742_S5_M1_356h.pdf
125742_S5_M3_guidelines-travel-red-zone-belgium.pdf
125742_S5_M3_requirements-travel-belgium.pdf
125742_S60_M1_356h.pdf
125742_S61_M1_356h.pdf
125742_S62_M1_356h.pdf
125742_S63_M1_356h.pdf
125742_S64_M1_356h.pdf
125742_S67_M1_356h.pdf
125742_S68_M1_356h.pdf
125742_S69_M1_356h.pdf
125742_S6_M1_356h.pdf
125742_S6_M5_5314_vr-vtn-10436.pdf
125742_S6_M5_5351_c4591001-sequencing-report.pdf
125742_S70_M1_356h.pdf
125742_S71_M1_356h.pdf
125742_S72_M1_356h.pdf
125742_S73_M1_356h.pdf
125742_S74_M1_356h.pdf
125742_S75_M1_356h.pdf
125742_S76_M1_356h.pdf
125742_S77_M1_356h.pdf
125742_S78_M1_356h.pdf
125742_S79_M1_356h.pdf
125742_S7_M1_356h.pdf
125742_S80_M1_356h.pdf
125742_S82_M1_356h.pdf
125742_S85_M1_356h.pdf
125742_S89_M1_356h.pdf
125742_S8_M1_356h.pdf
125742_S9_M1_356h.pdf
19736_S0185_M1_1571.pdf
19736_S0185_M1_cover.pdf
19736_S0185_M1_smsr-01dec2020-31dec2020.pdf
19736_S0214_M1_1571.pdf
19736_S0214_M1_cover.pdf
19736_S0214_M1_smsr-01jan2021-31jan2021.pdf
19736_S0243-smsr-01feb2021-28feb2021.pdf
19736_S0243_M1_1571.pdf
19736_S0243_M1_cover.pdf
19736_S0289_M1_1571.pdf
19736_S0289_M1_cover.pdf
19736_S0289_M1_smsr-01mar2021-31mar2021.pdf
19736_S0335-M1_smsr-01apr2021-29apr2021.pdf
19736_S0335_M1_1571.pdf
19736_S0335_M1_cover.pdf
19736_S0366_M1_1571.pdf
19736_S0366_M1_cover.pdf
19736_S0366_M1_smsr-30apr2021-31may2021.pdf
19736_S0369-dsur-22apr2020-21apr2021.pdf
19736_S0369_M1_1571.pdf
19736_S0369_M1_cover.pdf
19736_S0408_M1_1571.pdf
19736_S0408_M1_cover.pdf
19736_S0408_M1_smsr-01jun2021-30jun2021.pdf
19736_S0453_M1_1571.pdf
19736_S0453_M1_cover.pdf
19736_S0453_M1_smsr-01jul2021-31jul2021.pdf
19736_S0491_M1_1571.pdf
19736_S0491_M1_cover.pdf
19736_S0491_M1_smsr-01aug2021-31aug2021.pdf
1_PM_S85_BLA 125742-4_09-30-2021_Telecon_Advice.pdf
2_PM_S89_BLA 12572-5_09-10-2021_Telecon_Advice.pdf
3_PM_S89_BLA 125742-5_09-21-2021_Telecon_Other.pdf
4_PM_S89_BLA 125742-5_09-22-2021_Telecon_Advice.pdf
5_PM_S102_1_BLA 125742-8_09-21-2021_Memo_600.81 Lot Distributi.pdf
6_PM_S102_2_BLA 125742-8_09-21-2021_Memo_600.81 Lot Distributi.pdf
7_PM_S102_BLA 125742-8_09-29-2021_Letter_Waiver Request.pdf
8_PM_S102_BLA 125742-8_09-29-2021_Telecon_Other.pdf
9_PM_S105_BLA 125742-9_09-20-2021_Telecon_Advice.pdf
pd-production-110123
100A_BLA 125742-0_08-20-2021_Memo_Other.pdf100_BLA 125742-0_08-20-2021_Memo_Other.pdf
101_Courtesy Copy_BLA 125742-0_Pharmacovigilance Plan Review-Addendum Memo - COMIRNATY.pdf
102_1_BLA 125742-0_08-20-2021_Memo_Test Results.pdf
103_2_BLA 125742-0_08-20-2021_Memo_Test Results.pdf
105_BLA 125742-0_08-20-2021_Telecon_Advice_Information.pdf
107A_1_BLA 125742-0_08-20-2021_Telecon_Labeling via FAX_e.pdf
107_1_BLA 125742-0_08-20-2021_Telecon_Labeling via FAX_e.pdf
108_BLA 125742-0_08-21-2021_Inspection Related_Inspect.pdf
109_Courtesy Copy_2_BLA 125742-0_CMC Review Memo, August 21, 2021 - COMIRNATY.pdf
10_BLA 125742-11_12-22-2021_Memo_Committee Memo_CMC.pdf
110_1_Courtesy Copy_BLA 125742-0_Analytical Method Review Memo - COMIRNATY.pdf
111_BLA 125742-0_08-21-2021_Telecon_Advice.pdf
115_1_BLA 125742-0_08-21-2021_Telecon_Labeling via FAX_e.pdf
116_BLA 125742-0_08-21-2021_Inspection Related_Establi.pdf
117_2_BLA 125742-0_08-22-2021_Inspection Related_Inspect.pdf
118_1_BLA 125742-0_08-22-2021_Inspection Related_Inspect.pdf
119_Courtesy Copy_BLA 125742-0_Real World Evidence BLA Memo - COMIRNATY.pdf
120_Courtesy Copy_BLA 125742-0_CBER CMC BLA Review Memo - COMIRNATY.pdf
121_Courtesy Copy_BLA 125742-0_Employee-Officer List Memo, August 22, 2021 - COMIRNATY.pdf
122_BLA 125742-0_08-22-2021_Telecon_Labeling via FAX_e.pdf
123_Courtesy Copy_BLA 125742-0_August 23, 2021 Approval Letter - Comirnaty.pdf
124_BLA 125742-0_08-23-2021_Memo_Committee Memo_SBRA.pdf
125742-0_20219001.P0.pdf
125742-0_20219001.PC1.pdf
125742-0_20219002.P0.pdf
125742-0_20219002.PC1.pdf
125742-0_20219003.P0.pdf
125742-0_20219003.PC1.pdf
125742-0_20219004.P0.pdf
125742-0_20219005.P0.pdf
125742-0_20219006.P0.pdf
125742-0_20219006.PC1.pdf
125742-0_20219007.P0.pdf
125742-0_20219008.P0.pdf
125742-0_20219009.P0.pdf
125742_S111_M1_356h.pdf
125742_S111_M1_cover.pdf
125742_S111_M1_letter-of-authorization.pdf
125742_S112_M1_cover.pdf
125742_S112_S2_M3_32s2_control-critical-steps-manuf-process.pdf
125742_S116_M1_lab-1448-1-0.pdf
125742_S11_M1_cover.pdf
125742_S11_M1_response-25jun2021-cmc.pdf
125742_S11_M3_32r_21y513c2301-coa.pdf
125742_S11_M3_32r_21y513c2401-coa.pdf
125742_S11_M3_32r_21y513c2501-coa.pdf
125742_S11_M3_32r_677100p-1g-a-010-coa.pdf
125742_S11_M3_32r_677159p-1g-a-010-coa.pdf
125742_S11_M3_32r_677315o-1g-a-010-coa.pdf
125742_S11_M3_32r_677365-1g-a-010-coa.pdf
125742_S11_M3_32r_fc3181-coa.pdf
125742_S11_M3_32r_fc3184-coa.pdf
125742_S11_M3_32r_fd0809-coa.pdf
125742_S11_M3_32r_pf-07302048-dp-rm-coa.pdf
125742_S15_M1_cover.pdf
125742_S15_M1_response-16jul2021-lrp-samples.pdf
125742_S15_M3_32r_comirnaty-lot-release-16jul2021.pdf
125742_S17_M1_cover.pdf
125742_S17_M1_reponse-09jul2021.pdf
125742_S19_M1_cover.pdf
125742_S19_M1_response-02jul2021.pdf
125742_S1_M1_1.9.2_ped-studies-deferral-req.pdf
125742_S1_M1_1.9.4_ipsp-agreed.pdf
125742_S1_M1_financial-cert-3455.pdf
125742_S1_M1_financial-cert-summary.pdf
125742_S22_M1_cover.pdf
125742_S22_M1_response-16jul2021.pdf
125742_S25_M1_cover.pdf
125742_S25_M1_response-26jul2021.pdf
125742_S26_M1_andover-483-response.pdf
125742_S26_M1_cover.pdf
125742_S26_M1_fda-bnt-cl.pdf
125742_S2_M2_appendices-adv-agen-safe-val.pdf
125742_S2_M2_drug-product-description-composition-drug-prod.pdf
125742_S2_M2_drug-product-reference-stand-or-materials.pdf
125742_S2_M2_drug-product-stability.pdf
125742_S2_M2_introduction.pdf
125742_S2_M3_32a2_adventitious-agents-andover.pdf
125742_S2_M3_32a2_adventitious-agents-kalamazoo.pdf
125742_S2_M3_32a2_adventitious-agents-puurs.pdf
125742_S2_M3_32a3_excipient-information-excipients-alc-0159.pdf
125742_S2_M3_32a3_excipient-information-excipients-alc-0315.pdf
125742_S2_M3_32a3_excipient-information-nomenclature-alc-0159.pdf
125742_S2_M3_32a3_excipient-information-nomenclature-alc-0315.pdf
125742_S2_M3_32a3_excipient-information-structure-alc-0159.pdf
125742_S2_M3_32a3_excipient-information-structure-alc-0315.pdf
125742_S2_M3_32p1_description-and-composition.pdf
125742_S2_M3_32p2_pharmaceutical-development-introduction.pdf
125742_S2_M3_32p3_batch-formula.pdf
125742_S2_M3_32p3_control-critical-steps-in-process-mon-control-overview.pdf
125742_S2_M3_32p3_manufacturers.pdf
125742_S2_M3_32p4_excipients-human-animal.pdf
125742_S2_M3_32p4_novel-excipients.pdf
125742_S2_M3_32p52_analytical-procedure-cap-gel-electro.pdf
125742_S2_M3_32p52_analytical-procedure-endotoxin.pdf
125742_S2_M3_32p52_analytical-procedure-osmometry.pdf
125742_S2_M3_32p52_analytical-procedure-overview.pdf
125742_S2_M3_32p52_analytical-procedure-rt-pcr.pdf
125742_S2_M3_32p52_analytical-procedure-subvis-particulate-matter.pdf
125742_S2_M3_32p53_validation-analytical-procedure-cap-gel-electro.pdf
125742_S2_M3_32p53_validation-analytical-procedure-rt-pcr.pdf
125742_S2_M3_32p53_validation-analytical-procedures-overview.pdf
125742_S2_M3_32p5_batch-analyses.pdf
125742_S2_M3_32p5_specifications.pdf
125742_S2_M3_32p8_postapproval-stability.pdf
125742_S2_M3_32p8_stability-data-accelerated.pdf
125742_S2_M3_32p8_stability-data-long-term.pdf
125742_S2_M3_32p8_stability-data-photostability.pdf
125742_S2_M3_32p8_stability-data-thermal-stress-cycling.pdf
125742_S2_M3_32p8_stability-summary.pdf
125742_S2_M3_32r_reg-info-lot-release-documentation-package.pdf
125742_S2_M3_32r_reg-info-manufacturing-schedules.pdf
125742_S2_M3_32s2_control-critical-steps-manuf-process.pdf
125742_S2_M3_32s2_manufacturer.pdf
125742_S2_M3_32s4_analytical-procedure-overview.pdf
125742_S2_M3_32s4_specification.pdf
125742_S2_M3_32s4_validation-analyt-procedure-overview.pdf
125742_S30_M1_cover.pdf
125742_S30_M1_response-26jul2021.pdf
125742_S34_M1_cover.pdf
125742_S34_M1_response-03aug2021.pdf
125742_S34_M3_32a3_excipient-information-nomenclature-alc-0315.pdf
125742_S35_M1_cover.pdf
125742_S35_M1_response-04aug2021.pdf
125742_S36_M1_cover.pdf
125742_S36_M1_response-02aug2021.pdf
125742_S37_M1_cover.pdf
125742_S37_M1_response-05aug2021.pdf
125742_S40_M1_cover.pdf
125742_S40_M1_response-06aug2021.pdf
125742_S42_M1_cover.pdf
125742_S42_M1_response-04aug2021.pdf
125742_S42_M3_32r_comirnaty-lot-release-template.pdf
125742_S43_M1_cover.pdf
125742_S43_M1_response-05aug2021.pdf
125742_S43_M3_32p3_manufacturers.pdf
125742_S44_M1_cover.pdf
125742_S44_M1_response-10aug2021.pdf
125742_S47_M1_cover.pdf
125742_S47_M1_response-11aug2021.pdf
125742_S47_M3_32p3_manufacturer-diluent.pdf
125742_S48_M1_cover.pdf
125742_S48_M1_response-06aug2021.pdf
125742_S50_M1_cover.pdf
125742_S50_M1_response-13aug2021.pdf
125742_S50_M3_32r_comirnaty-lot-release-template.pdf
125742_S53_M1_cover.pdf
125742_S53_M1_response-13aug2021.pdf
125742_S55_M1_cover.pdf
125742_S55_M2_32p8_stability-data-accelerated.pdf
125742_S55_M2_drug-product-stability.pdf
125742_S55_M2_introduction.pdf
125742_S55_M3_32p8_stability-data-long-term.pdf
125742_S55_M3_32p8_stability-summary.pdf
125742_S56_M1_cover.pdf
125742_S56_M1_response-13aug2021.pdf
125742_S57_M1_cover.pdf
125742_S57_M1_response-16aug2021.pdf
125742_S57_M3_32p3_manufacturer-diluent.pdf
125742_S59_M1_cover.pdf
125742_S59_M1_response-17aug2021.pdf
125742_S5_M1_cover.pdf
125742_S5_M1_response-20-may-2021-facilities.pdf
125742_S60_M1_cover.pdf
125742_S60_M1_response-17aug2021.pdf
125742_S60_M3_32s2_control-critical-steps-manuf-process.pdf
125742_S61_M1_cover.pdf
125742_S61_M1_response-17aug2021.pdf
125742_S63_M1_cover.pdf
125742_S63_M1_response-18aug2021.pdf
125742_S80_M1_cover.pdf
125_BLA 125742-0_08-23-2021_Memo_Committee Memo_Toxico.pdf
127_Courtesy Copy_BLA 125742-0_CBER Sentinel Program Sufficiency Memo - COMIRNATY.pdf
128_BLA 125742-0_08-30-2021_Telecon_Other (1).pdf
129_BLA 125742-0_08-30-2021_Telecon_Other.pdf
131_Courtesy Copy_BLA 125742-0_Clinical Review Memo, August 23, 2021 - COMIRNATY.pdf
132_BLA 125742-0_09-13-2021_Memo_Committee Memo_Review (1).pdf
133_Courtesy Copy_BLA 125742-0_Benefit-Risk Assessment Review Memo - COMIRNATY.pdf
134_Courtesy Copy_BLA 125742-0_Toxicology Review - COMIRNATY.pdf
135_Courtesy Copy_BLA 125742-0_November 8, 2021_Summary Basis for Regulatory Action - COMIRNATY.pdf
136_BLA_125742_RNAcontent_InSupportTesting.pdf
137_BLA_125742_Endotoxin release tests lots FD7220, FE3592, FF2587.pdf
138_BLA_125742_STN125742 release test (pH) lots FD7220, FE3592, FF2587.pdf
15_BLA 125742-0_07-02-2021_Telecon_Information Reques.pdf
18_BLA 125742-0_07-09-2021_Telecon_Information Reques.pdf
1_BLA 125742-0_05-13-2021_Letter_Acknowledgement Let.pdf
1_IND_19736_185_23-Feb-2021_KERRY_KERRY__RO_.pdf
21_BLA 125742-0_07-15-2021_Letter_Filing Notification.pdf
25_1_BLA 125742-0_07-16-2021_Telecon_Information Reques.pdf
26_2_BLA 125742-0_07-16-2021_Telecon_Information Reques.pdf
27_BLA 125742-0_07-19-2021_Telecon_Advice.pdf
2_IND_19736_214_03-Mar-2021_KERRY_KERRY__RO_ .pdf
32_1_BLA 125742-0_07-26-2021_Telecon_Information Reques.pdf
33_1_BLA 125742-0_07-27-2021_Telecon_Information Reques.pdf
39_2_BLA 125742-0_08-02-2021_Telecon_Information Reques.pdf
3_BLA 125742-0_05-20-2021_Telecon_Information Reques.pdf
3_IND_19736_243_19-Mar-2021_KERRY_KERRY__RO_ .pdf
40_2_BLA 125742-0_08-03-2021_Telecon_Information Reques.pdf
42_1_BLA 125742-0_08-04-2021_Telecon_Information Reques.pdf
43A_STN 125742.0 4AUG21 LRP FU IR 2.pdf
44_3_BLA 125742-0_08-04-2021_Telecon_Information Reques.pdf
45_BLA 125742-0_08-05-2021_Telecon_Advice.pdf
46_2_BLA 125742-0_08-05-2021_Telecon_Information Reques.pdf
47_1_BLA 125742-0_08-05-2021_Telecon_Information Reques.pdf
49_BLA 125742-0_08-06-2021_Letter_UNII Code Notificat.pdf
4_IND_19736_289_27-Apr-2021_DEBORAH_DEBORAH__RC_.pdf
51_Courtesy Copy_BLA 125742-0_Pharmacovigilance Plan Review Memo - COMIRNATY.pdf
52_1_BLA 125742-0_08-06-2021_Telecon_Information Reques.pdf
53_2_BLA 125742-0_08-06-2021_Telecon_Information Reques.pdf
54_BLA 125742-0_08-09-2021_Inspection Related_Inspect.pdf.pdf
55_BLA 125742-0_08-09-2021_Telecon_Advice.pdf
58_2_BLA 125742-0_08-10-2021_Telecon_Information Reques.pdf
5_IND_19736_335_19-May-2021_DEBORAH_DEBORAH__RC_.pdf
60_BLA 125742-0_08-11-2021_Telecon_Information Reques.pdf
61_BLA 125742-0_08-11-2021_Telecon_Other.pdf
62_BLA 125742-0_08-12-2021_Memo_Committee Memo_Requir.pdf
64_Courtesy Copy_BLA 125742-0_Bioresearch Monitoring Discipline Review Memo, August 13, 2021 - COMIRNATY.pdf
65_BLA 125742-0_08-13-2021_Memo_Request For Complianc.pdf
66_4_BLA 125742-0_08-13-2021_Telecon_Information Reques.pdf
68A_5_BLA 125742-0_08-13-2021_Telecon_Information Reques.pdf
68B_5_BLA 125742-0_08-13-2021_Telecon_Information Reques.pdf
68_5_BLA 125742-0_08-13-2021_Telecon_Information Reques.pdf
6_IND_19736_366_24-Jun-2021_DEBORAH_DEBORAH__RO_.pdf
73_2_BLA 125742-0_08-16-2021_Telecon_Information Reques.pdf
74_3_BLA 125742-0_08-16-2021_Telecon_Information Reques.pdf
75_1_BLA 125742-0_08-16-2021_Telecon_Information Reques.pdf
79_3_BLA 125742-0_08-17-2021_Telecon_Information Reques.pdf
7_IND_19736_409_26-Jul-2021_DEBORAH_DEBORAH__RO_.pdf
81_1_BLA 125742-0_08-17-2021_Telecon_Information Reques.pdf
83_BLA 125742-0_08-17-2021_Telecon_Other.pdf
84_BLA 125742-0_08-18-2021_Memo_Committee Memo_Review.pdf
85_Courtesy Copy_BLA 125742-0_Statistical Review -- COMIRNATY.pdf
86_BLA 125742-0_08-18-2021_Telecon_Advice.pdf
87_3_BLA 125742-0_08-18-2021_Telecon_Information Reques.pdf
89_1_BLA 125742-0_08-18-2021_Telecon_Information Reques.pdf
8_1_BLA 125742-0_06-25-2021_Telecon_Information Reques.pdf
8_IND_19736_453_26-Aug-2021_DEBORAH_DEBORAH__RO_.pdf
92_BLA 125742-0_08-19-2021_Inspection Related_Inspect.pdf
93_Courtesy Copy_2_BLA 125742-0_Statistical Review - COMIRNATY.pdf
94_Courtesy Copy_1_BLA 125742-0_Statistical Review-COMIRNATY.pdf
95_BLA 125742-0_08-19-2021_Memo_Compliance Check Acce.pdf
99_BLA 125742-0_08-20-2021_Memo_Committee Memo_Labeli.pdf
9_IND_19736_491_29-Sep-2021_DEBORAH_DEBORAH__RO_ The Summary Monthly Safety Report has been reviewed. No furthe.pdf
FDA-CBER-2021-5683-1150835-1200874_Slipsheet.pdf
STN 124742_Courtesy Copy_Pfizer-BioNTech Comirnaty STN 125742 COVID-19 Vaccine Lot Notifications (2021).pdf
Screenshots er fra de første frigivene dokumenter